BOYLE’S LAW
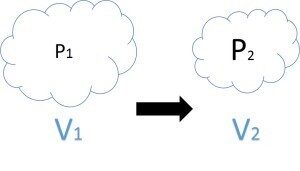 In drilling, the expansion of a gas due to its pressure decrease is often given in terms of Boyle’s Law. This law simply states that the expansion of a gas due to a pressure decrease is proportional to both the original volume of the gas and the amount of the pressure decrease. In mathematical terms: P1 * V1 = P2 * V2 or the pressure at the original state multiplied by the volume in the original state will be equal to the reduced pressure multiplied by the increased volume. When pressure becomes trapped in equipment deep downhole, it is of concern because that pressure can be released rather rapidly when the equipment is brought to the surface and actuated or disassembled. If the well in question happens to contain H2S in the wellbore fluids, this also becomes an issue of concern as a small amount of high pressure H2S gas when released can expand and displace a large volume of air as shown by Boyle’s Law.
In drilling, the expansion of a gas due to its pressure decrease is often given in terms of Boyle’s Law. This law simply states that the expansion of a gas due to a pressure decrease is proportional to both the original volume of the gas and the amount of the pressure decrease. In mathematical terms: P1 * V1 = P2 * V2 or the pressure at the original state multiplied by the volume in the original state will be equal to the reduced pressure multiplied by the increased volume. When pressure becomes trapped in equipment deep downhole, it is of concern because that pressure can be released rather rapidly when the equipment is brought to the surface and actuated or disassembled. If the well in question happens to contain H2S in the wellbore fluids, this also becomes an issue of concern as a small amount of high pressure H2S gas when released can expand and displace a large volume of air as shown by Boyle’s Law.
WIRELINE TOOLS
The first and most straightforward example is that of a pressure bomb, a device lowered downhole via wireline to collect and store wellbore fluids at wellbore pressure. When analyzing the sample in a lab environment, the expansion of the gas must be accounted for as the expanding gas and gas evolving from oil can rapidly displace the air. This expanding gas hazard becomes even more acute when the entrained fluids contain H2S. Due to Boyle’s Law expansion, even crudes considered marginally “sweet” could potentially render the atmosphere in a small space lethal due to expanding of depressurized hydrogen sulfide gasses.
ARTIFICIAL LIFT
The example above is unusual in that the worker is actively and routinely handling crude oil and gas so the procedures to protect the individual from actual exposure must be robust. In the next set of examples, we will examine the possibility in an arena where downhole fluids are not actively sought during surface activities such as completions and workover: that is within artificial lift equipment which is becoming used more in South Texas.
There are a few places that gasses and, more likely liquids, can become entrapped within an ESP. Care must be taken when breaking down the pump, shroud, and connections so that workers are not being exposed to possible wellbore fluids. H2S can be soluble in those fluids and released when disturbed.
Rod pump can surprise crews who are not used to this method of artificial lift as the variety of styles will have different pressure reactions during repair and maintenance activity. In the case of an insert pump, pulling rods can swab gas into the wellbore and up the tubing especially if the well is not quite dead. If the gas contains H2S then it can be directed towards the floorhand’s breathing zone. Conversely, a large bore tubing pump will have a much lower risk of swabbing while pulling rods because of the use of an on/off tool. In this case, there is a possibility of swabbing as well, but there should also be the ability to circulate a kill using the dump valve. Pulling tubing as opposed to rods is a different risk profile for gas influx because there is the standard BOP equipment installed with a FOSV/TIW available. The contributing factor that should give pause to an operation is if something is stuck as this can cause more problems to arise and greatly increase reaction time when those problems do arise. The rods and tubing repair example most seriously apparent is that of stripping rods. Being able to control a gas kick, let alone an H2S gas kick, while stripping rods requires an enhanced amount of training and pre-job planning. Also consider a pump that is locked up with the standing valve plugged at surface, there exists a possibility of trapped fluids entrained within the barrel.
OTHER LOCATIONS
There are three properties of H2S that can make it particularly pervasive in well servicing work. These properties include:
- Heavier than air
- Soluble in natural gas
- Soluble in water
The first seems to be the best known, the gas can settle within low spaces such as a pit or cellar. H2S has also been known to settle inside the mud cross on top of the back-pressure valve in lethal amounts. The next quality, that H2S is soluble in natural gas, which is lighter than air, can act as a carrier to lift the gas. When gas is released from a well, it can’t be assumed that hydrogen sulfide will immediately fall, it may lift along with the lighter weight of the natural gas. As we’ve written before, hydrogen sulfide concentrations may be very high in the tops of a tank to the solubility of light natural gas at that tank top. Also note that H2S is soluble in water, so produced water should be kept off of the skin. When water containing hydrogen sulfide soaks into the skin, it can cause irritation among other symptoms. The water can hold onto hydrogen sulfide without setting off the monitors until it becomes agitated.
Remember that this is not meant to be an exhaustive list of hazards in this location, but it should give the reader moment for pause to think about H2S in their own operations. Some major takeaways are that hydrogen sulfide and gas influxes in general are going to be more difficult when wellbore geometry becomes complex and components get stuck. Great care needs to be taken in these cases so that workers are trained to stop work and recognize any hazards that may be present.
Recommended Training: H2S Monitor San Antonio H2S
Notice: Article is provided as is and for informational use only. Eagle Ford Training San Antonio, its owners, instructors, and affiliates hereto referred as the company shall have no liability for and you shall defend, indemnify and hold harmless from and against any claim loss demand, liability, obligation, and expense based upon any injury or damage, spill or pollution, product liability, or any other loss that may occur. The liability for the use of information is solely yours notwithstanding any act of error or omission by the company.
